
A research team led by Professor Wang Zhiwei from our institution has published a paper in the journal Nature Communications titled “Strongly Coordinating Mediator Enables Single-Step Resource Recovery from Heavy Metal-Organic Complexes in Wastewater”. The paper proposes a strategy of strongly coordinating mediator-based electro-reduction (CMBER) for the single-step recovery of heavy metals from wastewater contaminated with heavy metal-organic complexes. In CMBER, amidoxime with superior coordinating abilities over traditional ligands is immobilized by an amidoximation reaction onto a flow-through electrode that concurrently functions as a filtration device. This unique process spontaneously captures heavy metal ions from their complexes without external energy input, followed by direct in situ electro-reduction for metal recovery. This approach eliminates the need to break heavy metal-organic complexes using potent oxidants generated at the anode, distinguishing this work from existing literature. The strategy creates a new dimension for cost-effective resource recovery and water purification.
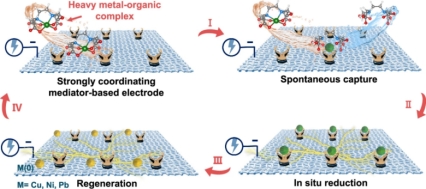
Fig.1. Schematic illustration of strongly coordinating mediator-based electroreduction
(CMBER) for heavy metal highly efficient recovery.
Heavy metal-organic complexes are a class of pollutants that are difficult to treat in industrial wastewater. Due to the strong affinity between heavy metal and organic ligands, the critical step in releasing heavy metal ions from heavy metalorganic complexes for subsequent reduction and recovery involves breaking the heavy metal-organic coordination bonds. Existing technologies, including advanced oxidation and electro-oxidation, generally depend on potent oxidants or substantial energy input to disrupt these complex structures, accomplishing this critical step inefficiently. These methods generally require a separate treatment process to recover the heavy metals. Therefore, creating an efficient method for heavy metal recovery without damaging the organic ligand represents a crucial challenge in addressing these limitations. Inspired by coordination exchange reactions, the paper proposed a strategy utilizing coordinating mediator-based electro-reduction for the efficient, single-step recovery of heavy metals from wastewater containing heavy metal-organic complexes (Fig. 1). By employing amidoxime, which has superior affinity for heavy metal ions compared to traditional ligands in complexes, this method facilitates the spontaneous capture of heavy metal ions directly from their complexes without the need for external energy. Subsequently, these metals captured by amidoxime groups can undergo direct in situ electro-reduction for recovery.
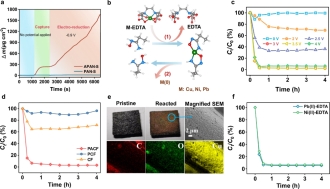
Fig. 2. Heavy metal-organic complexes capture and electrochemical recovery in CMBER. (a) Mass change from electrochemical quartz crystal microbalance with dissipation monitoring (EQCM-D) test. (b) The proposed pathways for Cu (II) recovery. (c) Effects of different voltages. (d) Comparison of Cu(II) recovery by different electrodes. (e) Digital photographs and the corresponding magnified scanning electron microscope (SEM) and energy dispersive spectrometer (EDS) images. (f) The recovery of Ni(II) and Pb(II).
Without applying any voltage, the amidoxime-functionalized electrode can spontaneously capture heavy metals until the sites are saturated. After applying a potential of −0.9 V vs. Ag/AgCl, the amidoxime-functionalized electrode continues to capture heavy metals, whereas the unfunctionalized electrode shows no significant heavy metal capture. These results suggest that the heavy metal ions in the heavy metal-organic complexes are first captured by the amidoxime group and then electro-reduced to their elemental form. Under an applied voltage of 3.0 V, the CMBER system achieved a 97.6% recovery rate of Cu(II), with the reduced copper uniformly distributed on the surface of carbon nanofibers. When a voltage of 4.2 V was applied, the amidoxime functionalized electrode was able to recover 93.2% of Ni(II) and 94.4% of Pb(II) from Ni(II)-EDTA and Pb(II)-EDTA, respectively. These results unequivocally demonstrate the ability of the amidoxime functionalized electrode to capture and recover heavy metals from heavy metal-organic complexes.
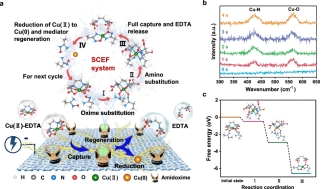
Fig. 3. Proposed pathways of the Cu recovery in CMBER. (a) Schematic of the proposed pathways. (b) In situ Raman spectra at different reaction times. (c) Energy barriers in different conformations.
The spontaneous capture and recovery mechanism of heavy metals by the amidoxime group was analyzed. Taking Cu(II)-EDTA as an example, in the stage Ⅰ, the Cu-O to Cu-N peak area ratio is 1.76, indicating that two carboxyl groups in Cu(II)-EDTA may be preferentially replaced by the -N-OH group of the amidoxime (ΔG = −0.51 eV), resulting in a four-coordinated state. In the stage Ⅱ, the ratio of Cu-O to Cu-N decreases to 0.48, indicating that the remaining two carboxyl groups in Cu(II)-EDTA may be replaced by the -NH2 group of the amidoxime (ΔG = −2.47 eV). In the stage Ⅲ, the ratio increases to 0.89, showing that the amidoxime group has fully captured Cu(II) (ΔG = −3.61 eV). In the stage Ⅳ, the captured Cu(II) is reduced to metallic copper, while the amidoxime sites are regenerated for the next cycle of Cu(II)-EDTA removal.
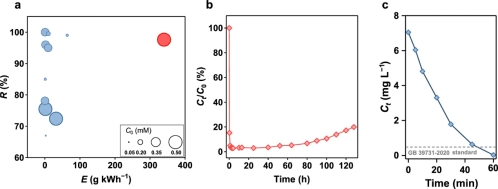
Fig. 4. Analysis of the applicability of CMBER. (a) Comparison on recovery and energy efficiency by different strategies. (b) The capacity during long-term test. (c) Recovery of Cu(II) from real Cu-containing chip manufacturing effluent water.
The energy efficiency of the CMBER system for Cu(II) recovery (measured as the amount of copper recovered per kilowatt-hour of electricity) is 340.1 g kWh−1, which is nearly five times higher than existing technologies. After operating for approximately 130 hours, the system still maintained about 80% Cu(II) recovery efficiency. Furthermore, the CMBER system effectively recovered copper from real industrial wastewater, and the effluent met the Chinese electronic industry’s water pollutant discharge standards (GB 39731-2020), indicating strong practical application prospects.
Shi Wei, a 2024 PhD graduate from our institution, is the first author of the paper. Co-authors include Dr. Li Jiayi, Dr. Gao Fei, and postdoctoral researchers Dr. Meng Lijun from our institution, as well as Associate Professor Xiao Su from the University of Illinois Urbana-Champaign. Professor Wang Zhiwei is the corresponding author of the paper. The research was funded by the National Natural Science Foundation of China.
Article Link: https://www.nature.com/articles/s41467-024-55174-1