Recently, the research team led by Professor Ma Jie from our institute has made significant advancements in ion desolvation regulation for enhancing capacitive deionization (CDI) desalination. The relevant findings have been published in the journal Advanced Functional Materials.
Electrochemical Na⁺ capture has emerged as a highly promising candidate for large-scale energy storage systems and brackish water purification technologies. The desolvation process of hydrated sodium ions (Na(H2O)x⁺) has been identified as the rate-determining step governing electrochemical kinetics. During the desalination process, irreversible water intercalation occupies active sites, thereby diminishing the storage capacity of Na⁺. Moreover, the high polarity of water molecules can lead to severe structural degradation and capacity attenuation of the electrode. MoS2 has garnered significant favor due to its abundant active sites and unique layered structure. However, owing to irreversible structural evolution and insufficient interlayer spacing, MoS2 exhibits poor cyclic stability and limited ion transport kinetics, which severely hinder its commercial applications. Notably, all these challenges are directly or indirectly associated with the ion desolvation process at the electrode/electrolyte interface. Therefore, the design of MoS2 electrode materials with regulated solvation behavior is of paramount importance for high-performance sodium-ion storage.
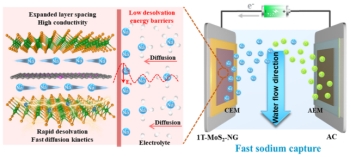
This study proposes a dual structural engineering strategy to construct stable nitrogen-doped layered carbon-intercalated 1T-phase MoS2 superlattice nanoflowers (1T-MoS2-NC) via phase transition and interlayer spacing expansion. This approach effectively modulates the desolvation process of Na(H2O)x⁺ and demonstrates exceptional CDI performance. The cation-π interactions induced by NC intercalation and the tunable interlayer structure reduces the desolvation energy of Na(H2O)x⁺. The abundant 1T metallic phase accelerates charge transfer while enhancing ion transport kinetics. Under the synergistic effect of the unique structural design and optimized desolvation capability, the 1T-MoS2-NC electrode exhibits superior salt adsorption capacity and remarkable long cycling life. Ex-situ Raman spectroscopy and XPS confirm the highly stable 1T phase during the charge-discharge process. EQCM-D and DFT calculations indicate that 1T-MoS2-NC possesses low desolvation activation energy and Na⁺ diffusion energy barriers. This work demonstrates the significant role of desolvation regulation in aqueous ion capture and provides new insights and directions for constructing high-performance sodium-storage electrodes. The study, with Ph.D. candidate Yifan Ren as first author and Tongji University as the corresponding affiliation, was supported by funding from the National Natural Science Foundation of China and the Shanghai Municipal Science and Technology Commission's Domestic Science and Technology Cooperation Program.
Article Link:: https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adfm.202502601